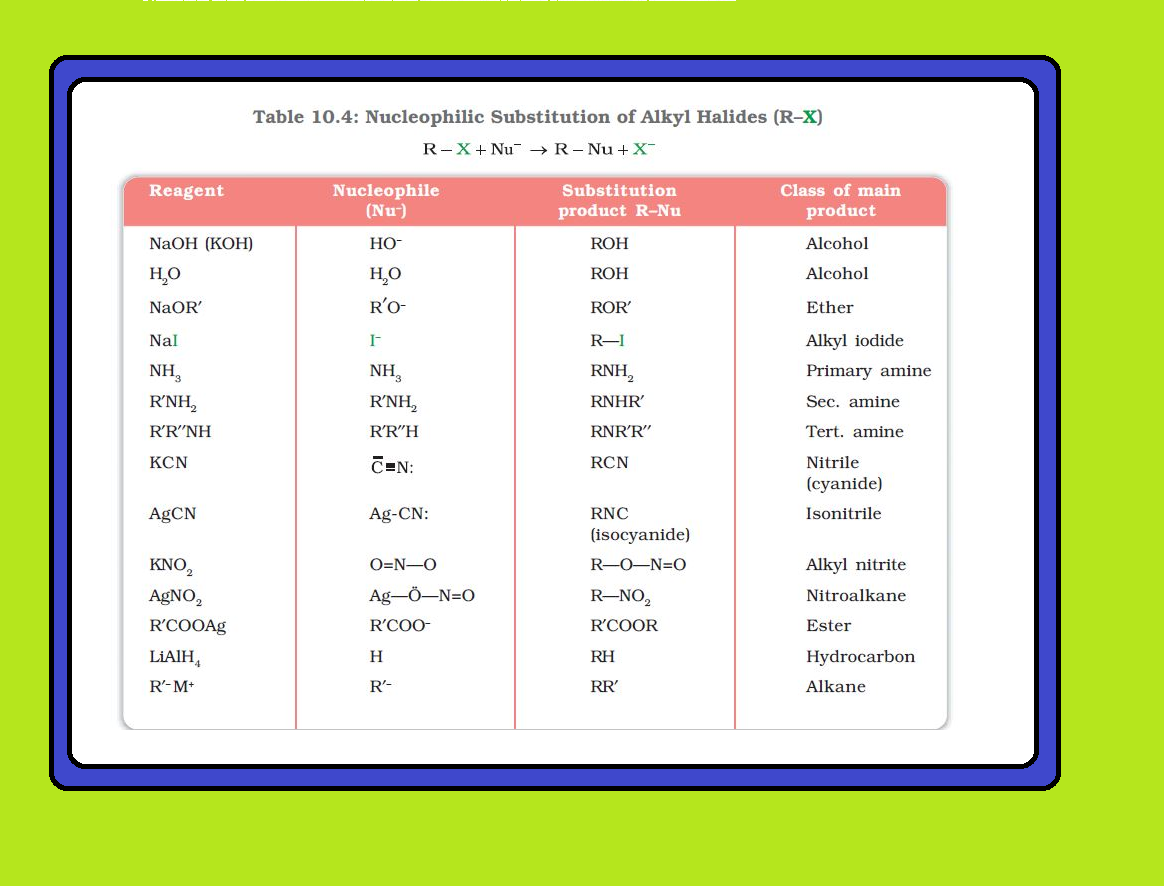
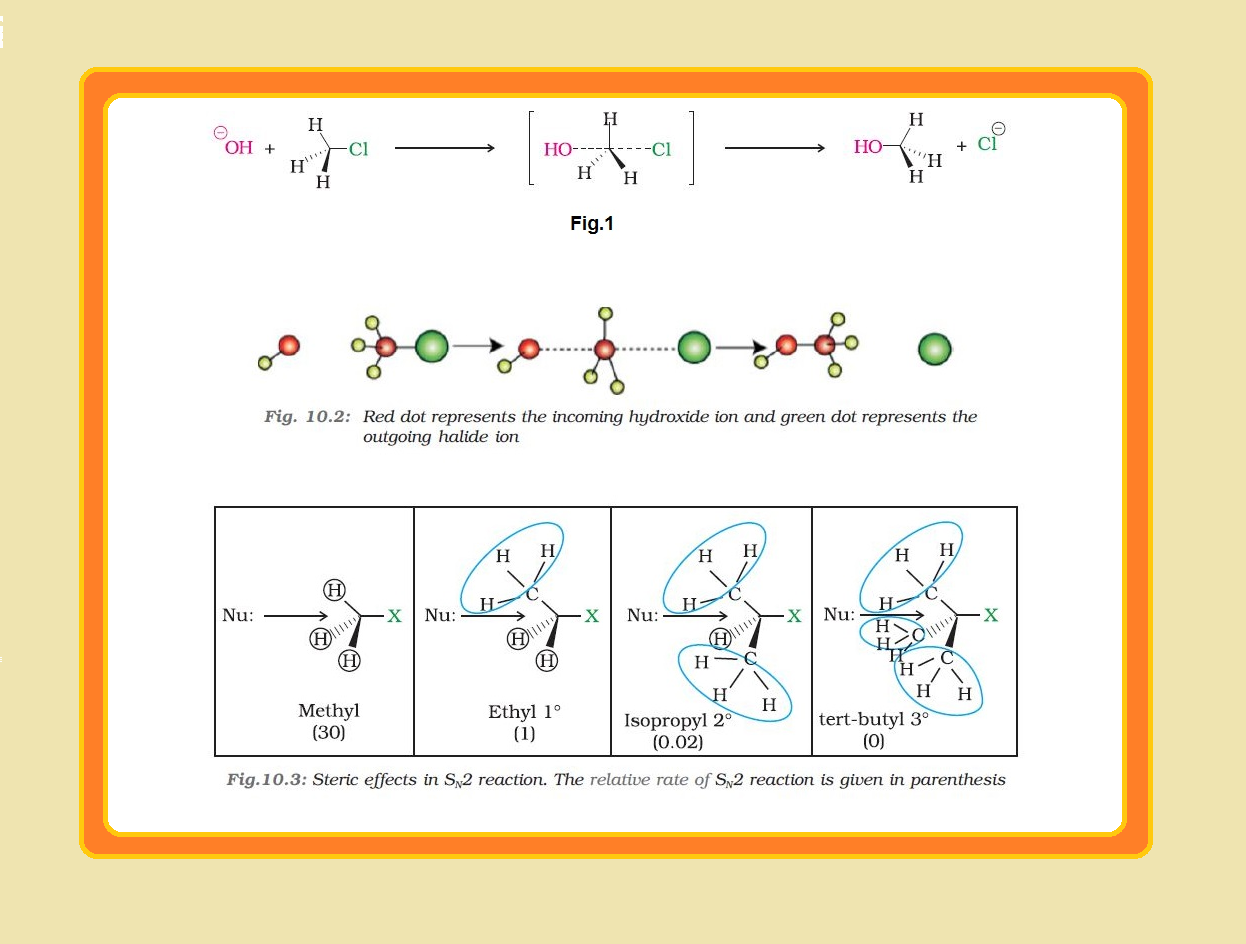
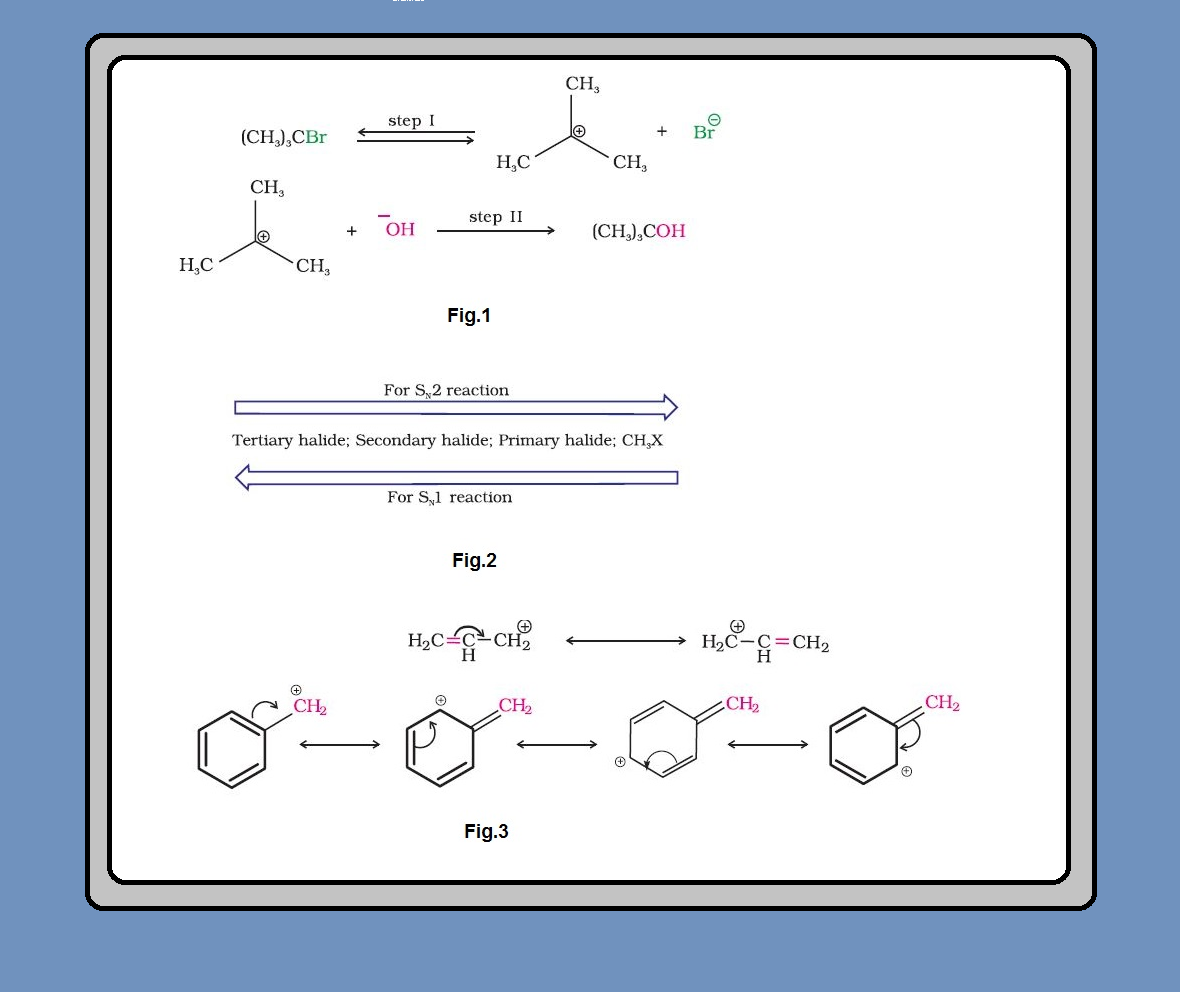
Melting and boiling points of haloalkanes and haloarenes :
`=>` Methyl chloride, methyl bromide, ethyl chloride and some chlorofluoromethanes are gases at room temperature.
`=>` Higher members are liquids or solids.
`=>` Molecules of organic halogen compounds are generally polar.
● Due to greater polarity as well as higher molecular mass as compared to the parent hydrocarbon, the intermolecular forces of attraction (dipole-dipole and van der Waals) are stronger in the halogen derivatives.
● That is why the boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass.
`=>` The attractions get stronger as the molecules get bigger in size and have more electrons.
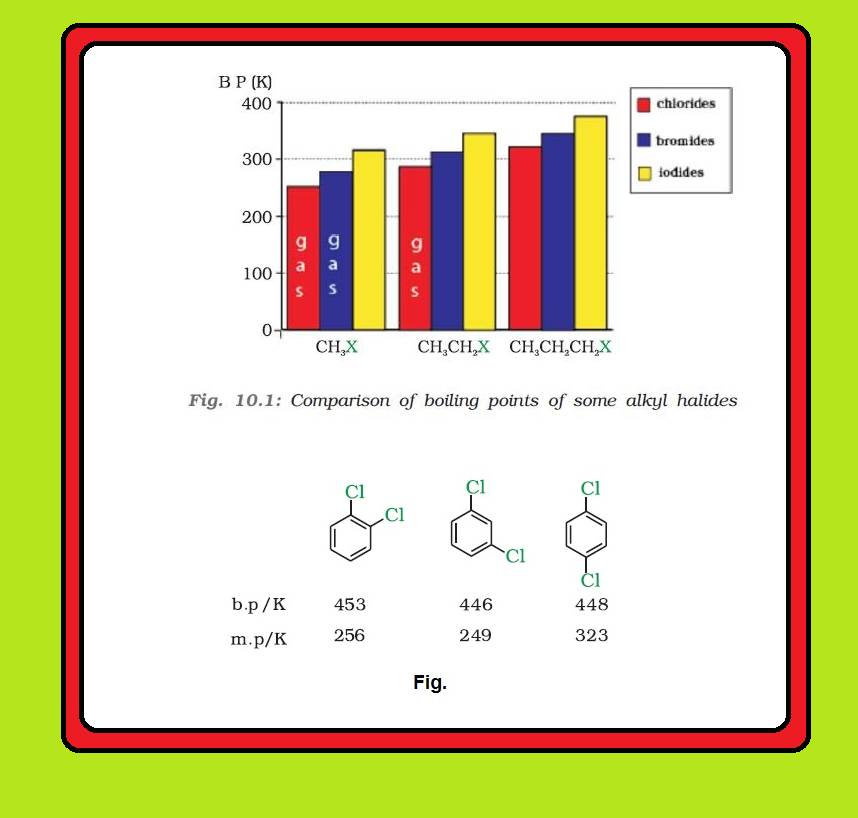
`=>` The pattern of variation of boiling points of different halides is depicted in Fig. 10.1.
`=>` For the same alkyl group, the boiling points of alkyl halides decrease in the order : `color{red}(RI > RBr > RCl > RF).`
● This is because with the increase in size and mass of halogen atom, the magnitude of van der Waal forces increases.
`=>` The boiling points of isomeric haloalkanes decrease with increase in branching.
● For example, 2-bromo-2-methylpropane has the lowest boiling point among the three isomers.
`color{red}(tt ( (, CH_3CH_2CH_2CH_2Br , CH_3CH_2underset(underset(Br)(|))CHCH_3 , H_3C- underset(underset(Br)(|)) overset(overset(CH_3)(|))C-CH_3 ) , (b.p//K , 375 , 364 , 346)))`
`=>` Boiling points of isomeric dihalobenzenes are very nearly the same.
`=>` However, the para-isomers are high melting as compared to their ortho- and meta-isomers.
● It is due to symmetry of para-isomers that fits in crystal lattice better as compared to ortho- and meta-isomers. See fig.
`=>` Higher members are liquids or solids.
`=>` Molecules of organic halogen compounds are generally polar.
● Due to greater polarity as well as higher molecular mass as compared to the parent hydrocarbon, the intermolecular forces of attraction (dipole-dipole and van der Waals) are stronger in the halogen derivatives.
● That is why the boiling points of chlorides, bromides and iodides are considerably higher than those of the hydrocarbons of comparable molecular mass.
`=>` The attractions get stronger as the molecules get bigger in size and have more electrons.
`=>` The pattern of variation of boiling points of different halides is depicted in Fig. 10.1.
`=>` For the same alkyl group, the boiling points of alkyl halides decrease in the order : `color{red}(RI > RBr > RCl > RF).`
● This is because with the increase in size and mass of halogen atom, the magnitude of van der Waal forces increases.
`=>` The boiling points of isomeric haloalkanes decrease with increase in branching.
● For example, 2-bromo-2-methylpropane has the lowest boiling point among the three isomers.
`color{red}(tt ( (, CH_3CH_2CH_2CH_2Br , CH_3CH_2underset(underset(Br)(|))CHCH_3 , H_3C- underset(underset(Br)(|)) overset(overset(CH_3)(|))C-CH_3 ) , (b.p//K , 375 , 364 , 346)))`
`=>` Boiling points of isomeric dihalobenzenes are very nearly the same.
`=>` However, the para-isomers are high melting as compared to their ortho- and meta-isomers.
● It is due to symmetry of para-isomers that fits in crystal lattice better as compared to ortho- and meta-isomers. See fig.